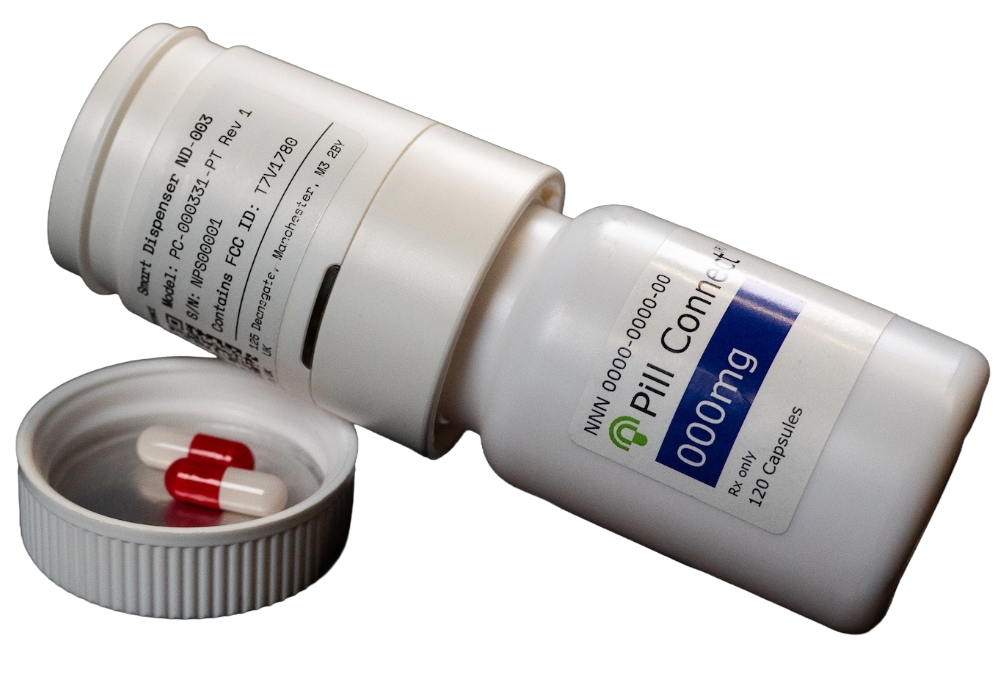
By providing objective, time-stamped adherence data as dosing occurs, Pill Connect supports trial teams in understanding and responding to real-world dosing behavior.
Equip site teams with objective adherence information to support discussions with participants during scheduled visits.
Identify missed or delayed doses sooner than periodic reviews of pill counts or diaries.
Interpret study data with greater clarity by understanding adherence patterns alongside other trial variables.
Maintain visibility into adherence when participants dose at home and site visits are less frequent.
Complement or replace methods that depend on self-reporting or retrospective review.
.jpg)
.jpg)
Can't find what you're looking for?
Yes. Pill Connect is suitable for use in clinical trials as well as launched drug programs where objective medication adherence data is required for patients or participants dosing at home.
Yes. Pill Connect supports decentralized and hybrid clinical trials by enabling medication adherence data to be collected remotely, without requiring participants to attend site visits solely for adherence monitoring.
Pill Connect provides objective, time-stamped dispensing data, reducing reliance on indirect adherence measures such as pill counts or self-reported patient diaries, which can be incomplete or inaccurate.
Pill Connect records dispensing events, including the date and time each individual pill is dispensed, as well as bottle swap events. This data can be used to support medication adherence analysis during a clinical trial.
The Pill Connect dispenser records events such as pill dispensing and bottle swaps. This data can be read by a smartphone and transmitted to remote monitoring platforms.
Yes. Adherence data recorded by Pill Connect can be transmitted via smartphone to remote monitoring platforms, allowing sites and CROs to review adherence information remotely during a clinical trial.
The standard Pill Connect dispenser works with capsule sizes 0 to 5 and a wide range of tablet sizes.
The standard dispenser fits bottle neck finishes 38-400 and 33-400. If a different bottle type is required, the dispenser can be customised to fit alternative neck finishes.
The dispenser has a battery life of up to 12 months in dispensing mode. It is powered by a sealed internal battery that cannot be replaced. During shipping, the device remains in a low-power sleep mode and is activated when first attached to a bottle.
Yes. The Pill Connect dispenser is certified to ISO 8317:2015 child-resistant and senior-friendly (CRSF) standards when used with 38 mm and 33 mm diameter neck bottles and it's 45mm protective top cap.
All materials that come into contact with pills are FDA-compliant, food-grade materials. Material compliance certificates can be provided on request.
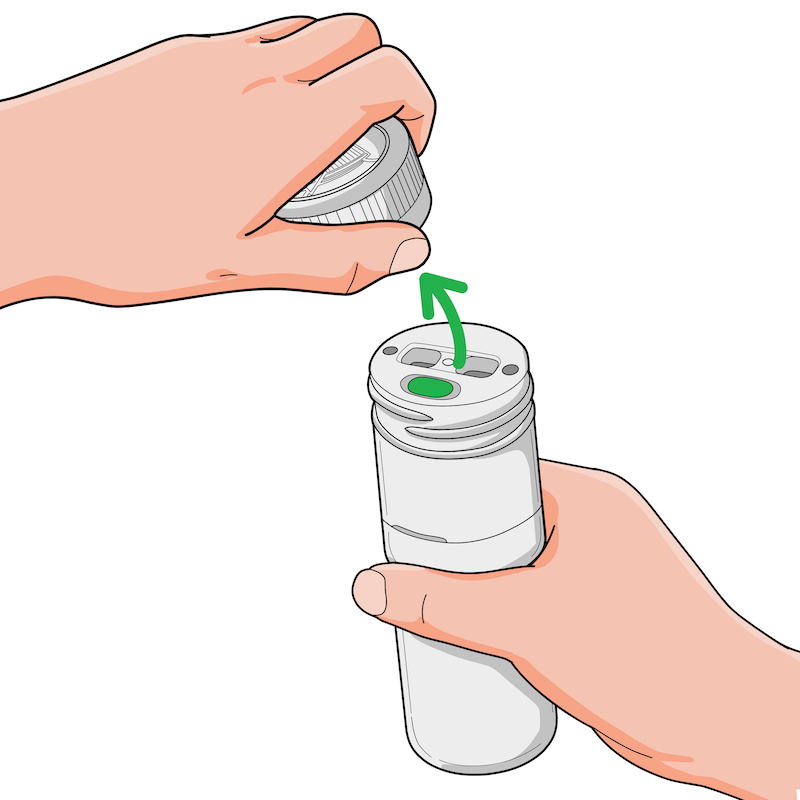
1. Remove the top cap
.jpeg)
2. Push the button to request a pill
.jpeg)
3. 1 pill is dispensed per button press, the date and time is recorded

4. Replace the top cap when finished